Latest Arthritis News
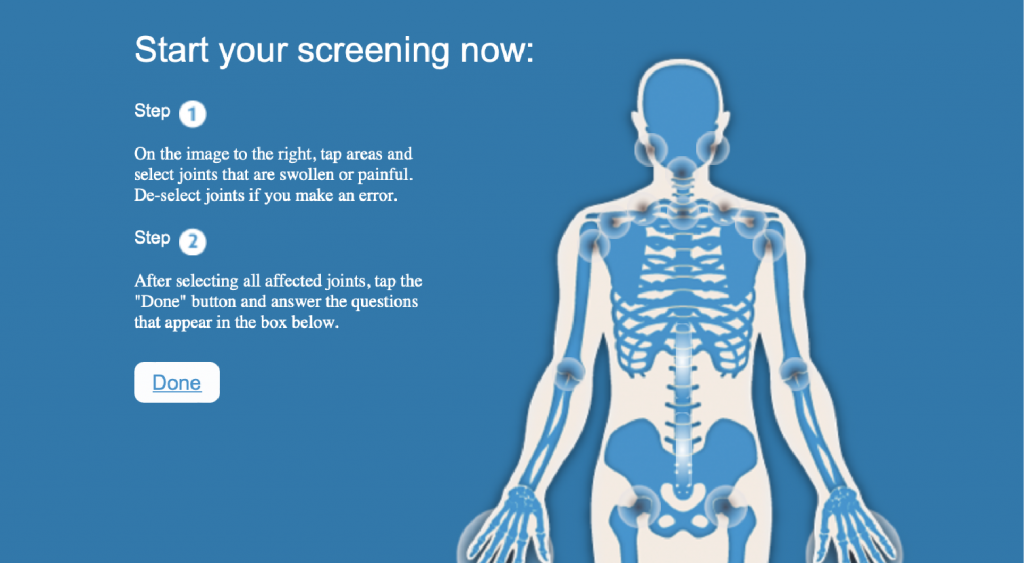
To help consumers identify arthritis, Arthritis Consumer Experts and Arthritis Research Canada developed an easy-to-use Arthritis Screening Exam.

Arthritis Consumer Experts National Survey on Artificial Intelligence and Arthritis Care

Arthritis Consumer Experts National Survey on Artificial Intelligence and Arthritis Care

May 10 is World Lupus Day!

#CRArthritis 2025: Interviews from Day 2

#CRArthritis 2025: Interviews from Day 1

Interview 1 – The impact of #CRArthritis for people living with arthritis

#CRArthritis 2025 – The Calgary edition!

People with late-onset rheumatoid arthritis experiences treatment delays

Fall Prevention Month: Aging with arthritis

National Pain Awareness Week: The pain of arthritis
Get to Know ABN
Led by Arthritis Consumer Experts (ACE), the Arthritis Broadcast Network (ABN) is a multi-media platform for the arthritis community to share news, information and stories about living well with arthritis. A proven, effective, popular source of arthritis patient information and education, Arthritis Broadcast Network has the most videos and views on a Canadian YouTube arthritis channel.
We acknowledge Arthritis Research Canada for serving in an advisory capacity for the scientific content of this website.
Subscribe to Arthritis Consumer Experts’ eNewsletter
Receive the latest research-based education, information, and advocacy on arthritis prevention, treatment, and research through our JointHealthTM insight newsletter and JointHealthTM express breaking news alerts. Offered in both English and French languages.
Subscribe to Arthritis Research Canada’s quarterly eNewsletter
Receive news and updates on arthritis research aimed at preventing arthritis, facilitating early diagnosis, finding better treatments, and improving quality of life for people with arthritis. Offered in both English and French languages.
Get to Know ABN
Led by Arthritis Consumer Experts (ACE), the Arthritis Broadcast Network (ABN) is a multi-media platform for the arthritis community to share news, information and stories about living well with arthritis. A proven, effective, popular source of arthritis patient information and education, Arthritis Broadcast Network has the most videos and views on a Canadian YouTube arthritis channel.
We acknowledge Arthritis Research Canada for serving in an advisory capacity for the scientific content of this website.
Subscribe to Arthritis Consumer Experts’ eNewsletter
Receive the latest research-based education, information, and advocacy on arthritis prevention, treatment, and research through our JointHealthTM insight newsletter and JointHealthTM express breaking news alerts. Offered in both English and French languages.
Subscribe to Arthritis Research Canada’s quarterly eNewsletter
Receive news and updates on arthritis research aimed at preventing arthritis, facilitating early diagnosis, finding better treatments, and improving quality of life for people with arthritis. Offered in both English and French languages.